Once a cleanroom facility is qualified, a routine environmental monitoring program must be designed, which includes identifying sampling locations, determining the frequency of monitoring, and conducting trend analysis. The environmental monitoring program should also outline procedures for facility cleaning, plate transfer, operator training, and failure investigation. A viable particle monitoring program should be established in all sterile manufacturing environments and documented.
Viable particle monitoring is designed to detect viable particles, which usually include bacteria and fungi. The program is designed to detect mesophilic and aerobic microorganisms that grow best at moderate temperatures. However, some manufacturing processes may require anaerobic monitoring.
Types of Viable Particle Monitoring
There are two different types of viable monitoring. Air monitoring and surface monitoring. Air monitoring can be conducted using both active and passive air sampling methods. Surface monitoring can be conducted using contact plates and swab sampling methods.
Active air sampling
The active air sampling method involves an air sampling device that measures the number of microorganisms in a given volume of air. Air samplers collect a defined quantity of air, typically one cubic meter (1000 liters), as specified by the guidelines. Surface air samplers are the most widely used type of air sampler.
These samplers draw air into the sampling head through a perforated sieve. The sampling head is mounted with an open agar plate before collecting the sample. The sieve has several holes. The diameter of the hole determines the air velocity and the number of microorganisms that enter the hole.
There is a probability that one or more cells enter the same hole and land on the agar surface at the same spot. These cells grow at the same spot and form one colony rather than two or more colonies.

Therefore, a correction factor must be applied to correct the count at the impaction points. Without correction, this could lead to an underestimation of the actual number of viable microorganisms in the sampled air.
The equation for the correction was developed by William Feller in 1968. The manufacturer of the air sampler will provide a Feller correction table depending on the number and the diameter of the holes. The microbiologist will use the correction table while reporting the results of active air monitoring. The result will be reported in CFU per cubic meter.
According to the Feller correction table, the statistical count is usually more than the observed count, as shown below.
| Observed Count | Statistical Count |
| 64 | 76 |
| 65 | 77 |
| 66 | 78 |
Passive air sampling
Unlike active air sampling, passive air monitoring does not require any specialized devices. It reveals the number of microorganisms present in the environment that are deposited on the agar surface without the application of any external force. The passive monitoring uses settle plates, which are standard 90-millimetre petri dishes containing culture media.
The settle plates are exposed to air at defined locations for no more than 4 hours during aseptic operations. In Grade A and B zones, monitoring should be conducted throughout aseptic operations. In this case, the plates should be replaced every 4 hours.
When the monitoring is performed for activities less than 4 hours, the results obtained should be extrapolated to 4 hours. For example, if three colonies are obtained for 1 hour of monitoring, the result will be reported as 12 CFU per 4 hours.
For grade C and D zones, the exposure time and monitoring frequency are based on quality risk assessment.
As the media is continuously exposed to the air, there is always a risk of desiccation, which will result in dryness and cracks in the media. The desiccation of media depends on the quantity of agar in the plate, the airflow rate, and the humidity. The 4-hour exposure time requires validation, which should consider the physical properties of the media and the recovery of microorganisms.
Surface Monitoring by Contact Plate Method
Surface monitoring is performed to check contamination levels on critical surface locations, including the floor, walls, and equipment, using surface contact plates. The surface contact plates are petri dishes with a diameter of 55 millimeters, providing a surface area of 25 centimeters squared. The agar in the contact plate is filled to a level above the edge. This convex part of the plate allows the agar to come into contact with the surface when it is pressed onto it, enabling the collection of the sample.
Surface sampling can also be performed for personnel working in Grades A and B zones. The sample can be collected from the sterile gown and hand gloves at the end of the activity. For personnel gown monitoring, 55-millimetre contact plates are used, and for sterile gloves, 90-millimetre plates are used for taking finger dabs. Personnel involved in aseptic operations in Grade A and B zones are required to be qualified in aseptic gowning. The qualification process involves surface sampling of gowning at various locations, including the forehead, arms, elbows, and gloves.
The surface contact method gives a replica of microorganisms present on a surface to be sampled. Therefore, the technique is also called “replicate organism detection and counting” (RODAC).

Surface Monitoring by Swab Sampling Method
Swabbing is another method of surface sampling. The swab sampling method is applied on irregular surfaces or on surfaces where contact plate sampling is not possible. Swabs have sterile cotton tips dipped into a pre-wetting medium or diluent. Sampling is performed by rotating the cotton tip to cover an area of approximately 24 to 30 square centimeters. After collecting the sample, the cotton swab is put back into the medium or diluent and then sent for testing.

Testing and Incubation of Viable Monitoring Samples
After the environmental monitoring samples are collected, the plates should be sent to the microbiology laboratory for incubation immediately. After completing the monitoring activity, it takes time to collect the plates, segregate them, wrap them, and transfer them to the quality control department. In ideal cases, the time between sampling at the last location and incubation should not be more than 3 hours, unless justified.
The viable monitoring plates from active, passive, and surface contact monitoring will be incubated at two different temperatures. Initially, the plates will be incubated at 20-25°C, and then they will be transferred to 30-35°C. At a temperature of 20-25°C, the incubation period should be at least 72 hours, and at a temperature of 30-35°C, it should be at least 48 hours. The temperature range of 20-25°C is suitable for supporting the growth of fungi, while the range of 30-35°C is suitable for supporting the growth of bacteria.
After receiving the swab sample, the diluent in which the swab is dipped will be tested by the membrane filtration method. In this method, the sample is filtered through a 0.45-micron filter, followed by rinsing to remove any remaining disinfectant residues. Then, the filter will be placed on an SCDA surface. The plates will be incubated at 20 to 25 degrees Celsius for not less than 72 hours, followed by incubation at 30 to 35 degrees Celsius for not less than 48 hours.
If the swab comes along with a nutrient medium, it can be directly incubated without any manipulation.
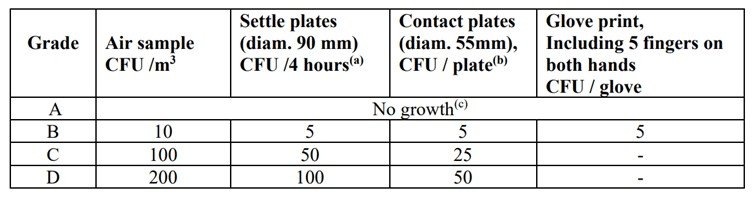
These are the specifications for viable monitoring in different grades, as outlined in the regulatory guidelines.
FAQs
Viable particles are microorganisms (such as bacteria, fungi, or yeast) that can grow and multiply under suitable conditions.
Viable particle monitoring is done using active air samplers, settle plates, contact plates, and swabs. These methods help collect microorganisms from air, surfaces, and equipment in cleanrooms.
Viable particles are living microorganisms that can grow when provided the right conditions. Non-viable particles are inert particles (like dust or fibers) that do not contain living organisms but can carry viable particles on their surfaces.
Frequency depends on the cleanroom grade and the operation. In Grade A/B areas, continuous or frequent monitoring during operations is required. Lower-grade areas (C/D) are monitored at defined intervals as per the environmental monitoring program.
If limits are exceeded, it triggers an investigation to identify root cause, assess product impact, and implement corrective actions. Trending, additional sampling, and review of cleanroom operations are part of the response.
Active air sampling pulls a measured volume of air through a device to capture microorganisms. Passive monitoring (e.g., settle plates) collects particles that naturally settle over time.
Read Biosafety Levels




