When a patient picks up a medicine, whether it is a tablet or an injection, they trust that it is safe for them. It’s not just about curing the problem. What matters most is that it doesn’t cause any adverse effects. It must not lead to any harmful side effects. But have you ever wondered what ensures this trust over time? That’s where stability studies in pharmaceuticals step in. These assessments are critical to maintaining drug integrity from production to the moment it reaches the patient’s hands.
Let’s explore what stability studies are, why they matter, and what ICH guidelines like ICH Q1A recommend.

What Are Stability Studies in Pharmaceuticals?
The primary objective of stability studies is to generate data that support the recommended storage conditions, shelf life, and retest period of drug substances, as well as the expiry date of drug products.
These studies also help in determining packaging requirements to protect the product from environmental hazards. Stability studies are essential not only for initial product registration but also during the life cycle of the product, including changes to formulation, manufacturing site, or process.
Global regulatory authorities mandate stability studies, and they provide the scientific basis for determining expiry dates, storage conditions, and packaging requirements. Without these studies, patients cannot determine whether the tablet they are consuming is still safe after a year.
ICH Stability Guidelines (ICH Q1A)
The International Council for Harmonisation (ICH) is a global organization that brings together regulatory authorities and the pharmaceutical industry to develop harmonized technical guidelines and standards for pharmaceutical products.
The ICH Q1A provides guidelines for the stability testing of new drug substances and products. It outlines the principles, protocols, and expectations that manufacturers must follow when designing and conducting stability studies.
What makes ICH stability guidelines so valuable is their harmonization of requirements across major markets like the US, EU, and Japan. Instead of running separate studies for each region, manufacturers can now design a single and globally acceptable stability program. This not only speeds up approvals but also ensures consistency in drug quality worldwide.
For those interested, you can explore the official ICH Q1A guideline to see the technical depth that goes into these protocols.
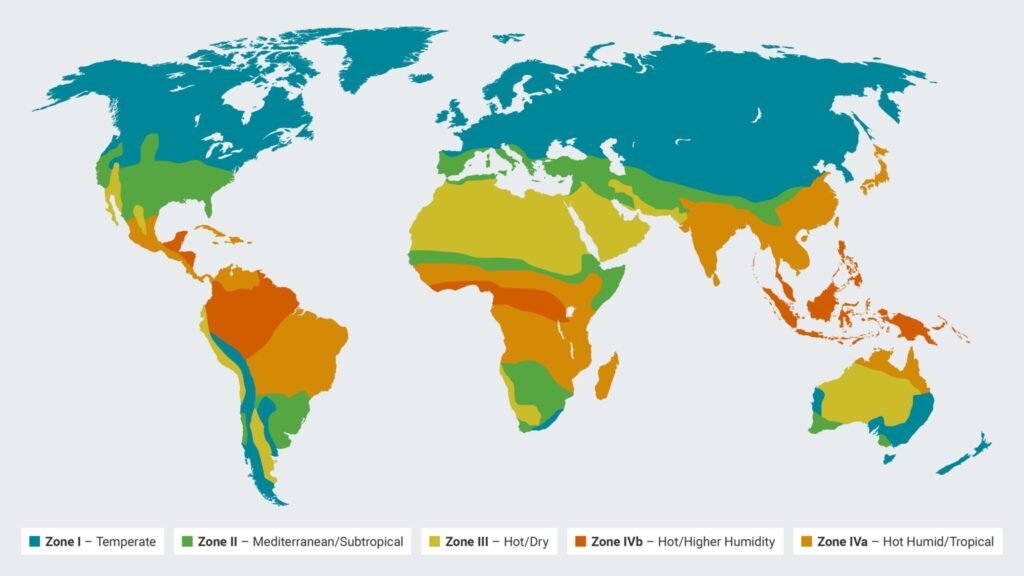
What are Climatic Zones?
Pharmaceutical products are distributed globally, and they may be exposed to very different environmental conditions depending on where they’re stored and used. To standardize stability testing, the World Health Organization (WHO) and the International Council for Harmonization (ICH) define climatic zones based on the average temperatures and humidity in different regions worldwide.
| Climatic Zone | Typical Conditions | Example Regions |
| Zone I | Temperate | Northern Europe, Canada |
| Zone II | Subtropical / Mediterranean | Southern Europe, USA (some parts) |
| Zone III | Hot dry | Parts of Australia, Mexico |
| Zone IVa | Hot humid | Parts of Asia, Latin America |
| Zone IVb | Hot and very humid | Southeast Asia, parts of India, and Africa |
Study Design
According to ICH Q1A (R2), a stability study should be carefully planned and documented. It begins with selecting appropriate batches for testing. It requires data from at least three primary batches. These batches should be manufactured using the same process intended for commercial production and should have the same formulation, packaged in the same container-closure system as proposed for marketing. The study must include long-term, intermediate, and accelerated conditions.
Stability Study Conditions
In stability studies, different products and markets demand different conditions. Here’s a simple table summarizing standard ICH stability conditions:
| Study Type | Temperature | Humidity | Duration |
| Long-term (Zone II) | 25°C ± 2°C | 60% RH ± 5% | 12-60 months |
| Intermediate | 30°C ± 2°C | 65% RH ± 5% | 6 months |
| Accelerated | 40°C ± 2°C | 75% RH ± 5% | 6 months |
These conditions are designed to mimic real-world environments (long-term) or overstress them (accelerated) to predict product stability faster.
Types of Stability Studies
1. Real-time stability studies
Monitor the product over time under recommended storage conditions. The product (e.g., tablet, injection, cream) is stored at the exact conditions that are recommended on its label — like temperature, humidity, and light protection — for the same duration as its intended shelf life.
For example:
If a medicine’s label says “Store at 25°C / 60% RH”, the stability study will store test batches at 25°C / 60% RH for 12, 24, or 36 months, depending on the intended shelf life.
The quality control team regularly tests these samples at specific intervals (e.g., 0, 3, 6, 9, 12 months, and so on) to check whether the product’s potency, safety, and quality remain acceptable.
2. Accelerated stability studies
The accelerated studies use harsher conditions to predict long-term stability. It is about intentionally storing a drug product at higher temperatures and humidity levels than it would normally experience during storage.
The accelerated conditions intentionally expose the product to higher temperatures and humidity to speed up the degradation process. This helps companies predict how the product behaves over time and allows them to make faster decisions on shelf life, packaging, and storage recommendations without waiting for years of data.
For example, let’s say your medicine is intended to be stored at 25°C and 60% relative humidity (normal storage conditions). In an accelerated stability study, you might store it at 40°C / 75% RH (hot and very humid)
This is much harsher than what the product would face in real life.
3. Stress testing
Stress testing is also called forced degradation testing. It is a type of stability study where the drug substance or product is deliberately exposed to extreme conditions extreme heat, UV light, humidity, etc.). The goal is not to see if the product survives these harsh conditions. Instead, stress testing is done to understand how and why the drug degrades and establish the drug’s degradation pathways.
4. In-use stability studies
In-use stability studies are designed to assess the stability of a pharmaceutical product after it has been opened, reconstituted, or diluted, but before it is fully administered to the patient.
Many medicines, especially multi-dose vials, injectables, oral suspensions, and reconstituted powders, cannot be consumed all at once. Instead, these products may be opened and stored (e.g., insulin vials, eye drops) for a certain period or used for multiple patients (multidose vaccines).
In such cases, the product will be exposed to air, and there is a risk of microbial contamination. As a result, the medicine may degrade more quickly.
The in-use stability study ensures that each dose remains safe, potent, and free from harmful degradation between first opening and final use. They define how long a product is good for after opening, mixing, or diluting — and under what storage conditions (e.g., “use within 7 days after reconstitution, store at 2-8°C”).
Apart from these four types, these studies cover various specialized studies, each with a distinct purpose.
| Type of Stability Study | Purpose |
| Intermediate Stability | To evaluate stability under conditions between long-term and accelerated, when accelerated testing fails. |
| Photostability Testing (ICH Q1B) | To assess the effect of light exposure on product quality. |
| Shelf-life Extension Studies | To justify extending the approved shelf life using additional or ongoing stability data. |
| Shipping / Transportation Stability | To assess stability after exposure to transport-related stress like temperature excursions. |
ICH Q1A vs. Other Regional Requirements
As we discussed, the ICH Q1A offers a globally harmonized framework for stability testing. However, there are subtle differences that exist between regions due to climatic conditions, regulatory philosophies, and public health priorities of that country.
For example, Brazil’s ANVISA and India’s CDSCO often require stability studies that go beyond standard ICH conditions. They emphasize additional requirements, such as Zone IVb conditions (30°C ± 2°C / 75% RH ± 5%), for hot and humid climates. The medicines in these regions are exposed to hot and very humid climates.
A company that designs its stability study only around 25°C / 60% RH long-term conditions (common in ICH Q1A) might not meet the requirements of countries in Zone IVb unless additional data is generated.
Documentation
ICH guidelines highlight the importance of thorough documentation. Stability protocols must be predefined, and all data, observations, and deviations must be recorded in detail. The data generated should be scientifically robust, reproducible, and capable of withstanding regulatory inspection. Proper documentation ensures traceability and supports regulatory submissions, inspections, and audits.
Also read Top 5 Trends Shaping the Pharmaceutical Industry in 2025




