In pharmaceutical manufacturing, particularly for parenteral preparations, equipment and primary packaging materials must be free from pyrogens. Pyrogens, especially lipopolysaccharides (endotoxins), can induce fever if introduced into the body. This article provides a complete overview of the depyrogenation methods and validation processes that ensure the protection of the finished product.

What is Depyrogenation?
Depyrogenation refers to the removal or inactivation of pyrogens, with a particular focus on lipopolysaccharides — the most potent and challenging to eliminate. This critical process applies to any item that comes into direct contact with sterile pharmaceutical products.
Depyrogenation Processes
Dry Heat Treatment
Dry heat treatment is the most common and reliable depyrogenation method for glassware and other heat-stable items. In any depyrogenation cycle, the items are usually subjected to a temperature of at least 250°C for a minimum of 30 minutes. Alternatively, the method can be validated at different temperature and time combinations depending on the type of product. But it must be a minimum of 180°C for 3 hours.
The process takes place in ovens or tunnels equipped with forced air circulation. To ensure consistent effectiveness, critical parameters like time, temperature, and belt speed (if applicable) are continuously monitored at the most challenging locations that struggle to achieve the required temperature during equipment qualification.
Validation of the dry heat process involves placing endotoxin indicators at these critical points to verify the efficacy of depyrogenation.
Alternative Depyrogenation Methods
When heat treatment isn’t feasible, other methods can be employed:
Physical Treatment: Items may be rinsed with pharmaceutical-grade water, such as Water for Injections (WFI), followed by drying.
Chemical Treatment: Strong oxidizing, alkylating, or reducing agents in liquid or vapor form (with or without heat) can remove pyrogens. A thorough final rinse with WFI is performed, and residual chemicals are verified through testing.
Like dry heat, these methods require validation using endotoxin indicators to confirm the removal of endotoxins.
Endotoxin Indicators: Essential Tools for Validation
Endotoxin indicators are made of purified bacterial endotoxins (lipopolysaccharides). Users must be aware of the following details of the indicators:
| Attribute | Details Required by User |
| Manufacturer name | Must be provided |
| Indicator composition | If applicable |
| Microorganism from which the endotoxin is isolated | Required (with the culture collection number, if available) |
| Specific activity of the endotoxin | Required (in IU / gram or mL or container) |
| Storage conditions | Must be known |
| Expiry date | Clearly stated |
| Batch number | Provided for traceability |
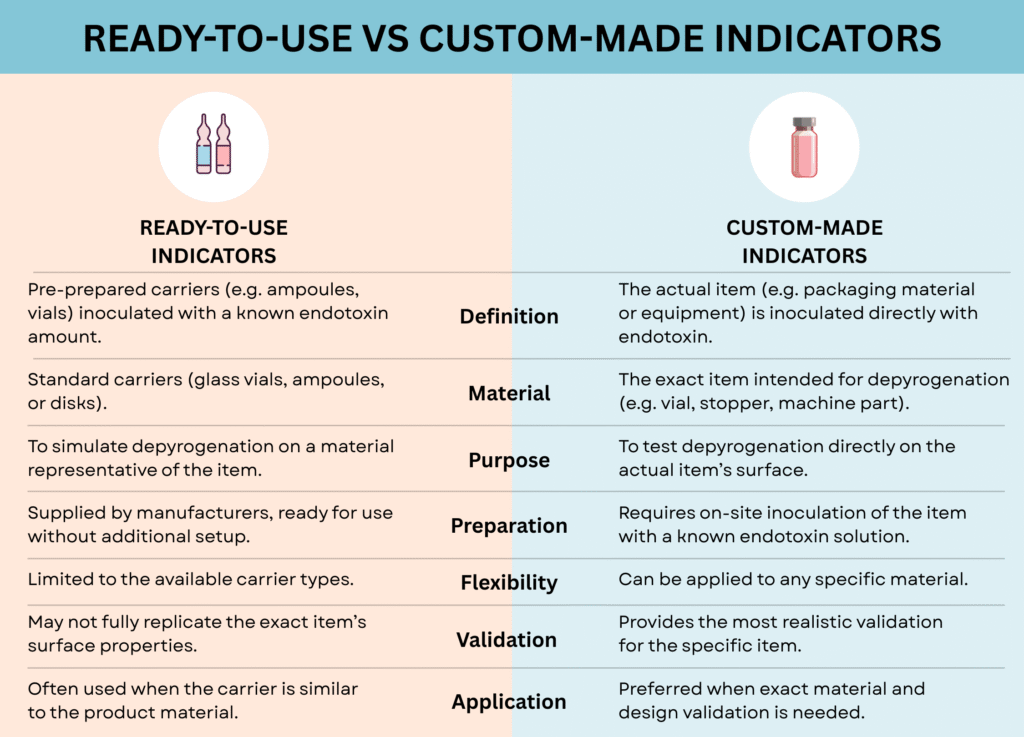
Depending on the item being depyrogenated (e.g., glass, plastic, stainless steel), a suitable endotoxin indicator—ready-to-use or custom-made must be selected.
Ready-to-Use Indicators
These are carriers like vials or ampoules inoculated with a known endotoxin level and used in the validation. The carrier material should represent the item being depyrogenated to ensure effective depyrogenation.
This is important because different materials react differently to depyrogenation processes. For example, heat penetrates glass and metal differently than it does in plastic. The surface properties, like smoothness and porosity, can affect how well pyrogens are removed.
So, using a carrier that closely mimics the actual item ensures that the depyrogenation process is truly effective for that specific material in real production conditions.
Custom-Made Indicators
Custom-made indicators involve directly inoculating the item (e.g., packaging material) with a known endotoxin solution. Once dried, these are processed to assess endotoxin reduction.
Instead of using a standard carrier (like a vial or ampoule pre-loaded with endotoxin), you take the actual item you want to validate — for example, a syringe, vial, stopper, or machine part — and you apply (inoculate) it with a measured amount of endotoxin (usually a solution of known concentration).
Once the endotoxin solution dries on the item’s surface, the item undergoes the depyrogenation process.
After depyrogenation, you measure the amount of endotoxin remaining on the item. By comparing the amount before and after treatment, you assess how effective the depyrogenation process was in reducing or eliminating endotoxin.
Custom-made indicators provide a highly realistic validation because they test depyrogenation directly on the real material and shape of the item that will be used in production. This ensures that the depyrogenation process works as intended on those exact surfaces, which may behave differently than standard carriers.
Test Procedures for Depyrogenation Validation
Endotoxin Recovery Test
Before you start testing whether your depyrogenation process works, you first need to check if you can accurately detect and measure the endotoxins on your indicators (ready-to-use or custom-made). This check is called the endotoxin recovery test.
It confirms that the endotoxin applied on the item can be appropriately measured using your chosen test method. In other words, it confirms that there is nothing in the material or the test process that blocks the endotoxin so that you can measure it properly.
If your recovery rate is too low, you may think your depyrogenation process worked well, but in reality, your test couldn’t recover it properly.
You won’t be able to demonstrate the required 3-log reduction, which means a 99.9% decrease in endotoxin levels. In such cases, you must start with a higher initial amount of endotoxin on the indicator.
This ensures that, even after losses due to poor recovery, you still have sufficient measurable endotoxin to accurately assess the effectiveness of your depyrogenation process.
Endotoxin Reduction Test
The amount of endotoxin must be measured before the depyrogenation process and again after the process to estimate the residual endotoxin level.
Then, compare the endotoxin level before treatment and after treatment using a validated test method. The difference is expressed as a log reduction.
Example in numbers:
If you started with 10,000 IU, and after depyrogenation, you have 10 IU left:
Reduction: log10(10,000) – log10(10) = 4 – 1 = 3 log reduction

Acceptance Criteria
The process is considered valid if it achieves at least a 3.0 log reduction in recoverable endotoxin.
Depyrogenation plays a vital role in the pharmaceutical manufacturing of parenteral products. Through dry heat or alternative techniques, thorough validation ensures that all items in direct contact with sterile medicines are free from harmful pyrogens and safe for patients.
Download Ph. Eur. chapter 5.1.12 (Depyrogenation) for your reference.




