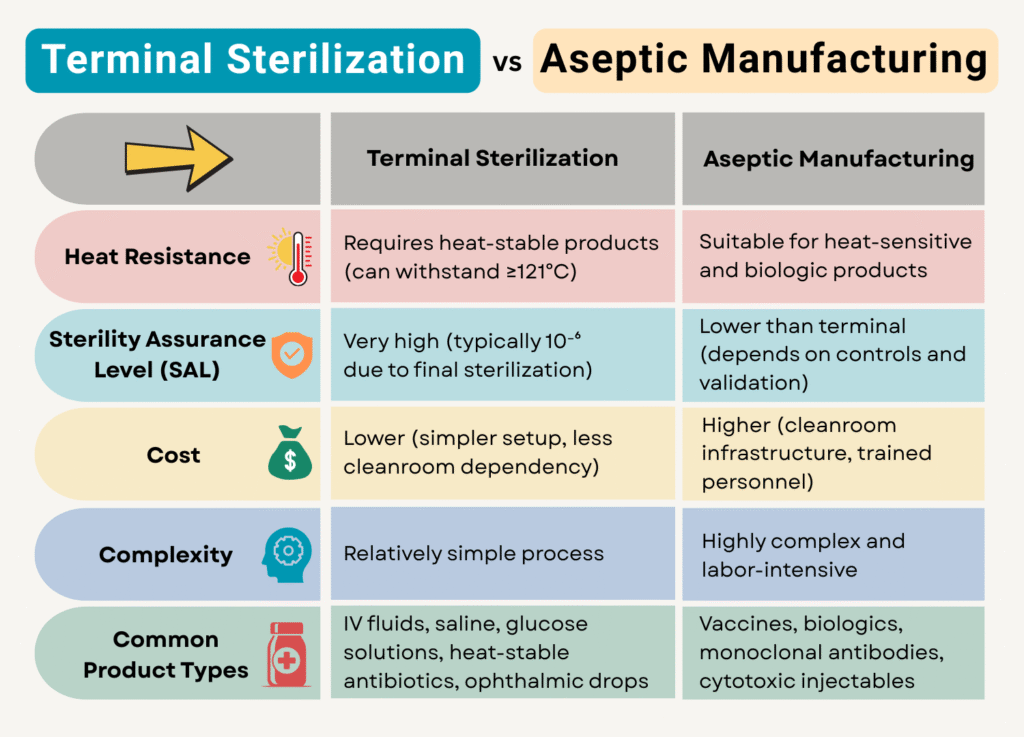
Sterile pharmaceutical products must be free from microbial contamination to ensure patient safety. The sterility of the product can be achieved by two methods: terminal sterilization and aseptic manufacturing.
Between these two methods, terminal sterilization is more reliable. However, not all products can withstand this process.
Although aseptic manufacturing is more complex than terminal sterilization, it becomes necessary for heat-sensitive formulations.
Let us explore when these methods are used, their differences, and why one cannot replace the other.
What is Terminal Sterilization?
Terminal sterilization involves subjecting the final, sealed pharmaceutical product to a sterilization process. The most common method of sterilization is by steam sterilization (autoclaving) at high temperatures (typically 121°C or higher). This method is highly effective because it ensures that both the product and its container are sterile, thereby minimizing the risk of contamination.
Despite its advantages, terminal sterilization cannot be used for all pharmaceutical products. The primary reason is heat sensitivity. Many biologics, protein-based drugs, vaccines, and certain parenteral formulations degrade when exposed to high temperatures.
Exposure to high temperatures causes the following:
- Chemical degradation – Active pharmaceutical ingredients (APIs) may break down, reducing potency.
- Physical instability – Excipients or the drug itself may undergo undesirable changes like precipitation and denaturation.
- Failed stability studies – Even if the product appears to be stable initially, long-term storage may cause degradation, making the product unsafe or ineffective.
For such products, aseptic manufacturing becomes the only viable option.

Products Suitable for Terminal Sterilization
1. Aqueous Injectable Solutions
- Sodium chloride injection
- Dextrose injection
- Ringer’s lactate solution
- Water for injection (bulk containers)
- Certain parenteral nutrition solutions (if thermally stable)
2. Ophthalmic Solutions
- Artificial tears
- Certain antibiotic eye drops (e.g., tobramycin, gentamicin) if stable
3. Irrigation Fluids
- Normal saline irrigation
- Sterile water for irrigation
- Glycine or sorbitol irrigation solutions (if heat-stable)
4. Oil-Based Injectables (only if shown to be thermally stable)
- Some vitamin injections in oil (e.g., vitamin A or D in peanut/sesame oil)
- Hormonal injectables in oil (e.g., progesterone in castor oil)
5. Non-Aqueous Solvents
- Some alcohol-based injectable solutions
- Heat-stable solvents used in depot formulations
6. Ophthalmic Ointments (in suitable packaging)
- Certain preservative-free formulations
- Ointments that do not degrade or lose viscosity under heat
7. Plastic or Glass Containers (If Heat Resistant)
- Pre-filled syringes (suitable for autoclaving)
- Large volume parenteral (LVP) bottles and bags
8. Surgical and Hospital Use Products
- Empty sterile saline bags
- Flush solutions for catheters
- Heat-stable diluents for reconstitution
Common Terminal Sterilization Methods
- Steam Sterilization (Autoclaving): 121°C+ for liquids/glass.
- Dry Heat: 160–180°C for powders/glassware.
- Gamma Irradiation: For pre-packaged devices.
- Ethylene Oxide (EtO): Heat-sensitive plastics.
Key Requirement: The product and packaging must withstand sterilization conditions without degradation.
What is Aseptic Manufacturing?
Aseptic manufacturing in the pharmaceutical industry is a sterile production process where drug products are prepared and filled into containers in a strictly controlled and sterile environment. It ensures that no contamination occurs at any stage.
Unlike terminal sterilization, where the final product is sterilized in its sealed container, Aseptic manufacturing involves sterilizing individual components (such as vials, stoppers, and filling equipment) separately and then assembling them in a controlled, sterile environment. The drug product is filtered through a sterilizing-grade filter (0.2 µm) to remove microbes before filling into pre-sterilized containers.
Key Requirements for Aseptic Processing
Aseptic manufacturing demands stringent controls on the environment. The filling zone must meet ISO Class 5 (Grade A) conditions, meaning fewer than 3,520 particles (≥0.5 µm) per cubic meter. Grade A zones are typically surrounded by Grade B (for conventional cleanrooms) or can be replaced within an isolator (Grade A) with Grade C/D background.
All primary contact materials, such as vials, stoppers, and syringes, must be sterilized prior to the filling. Operators must follow strict aseptic techniques and good manufacturing practices (GMP) to prevent contamination.
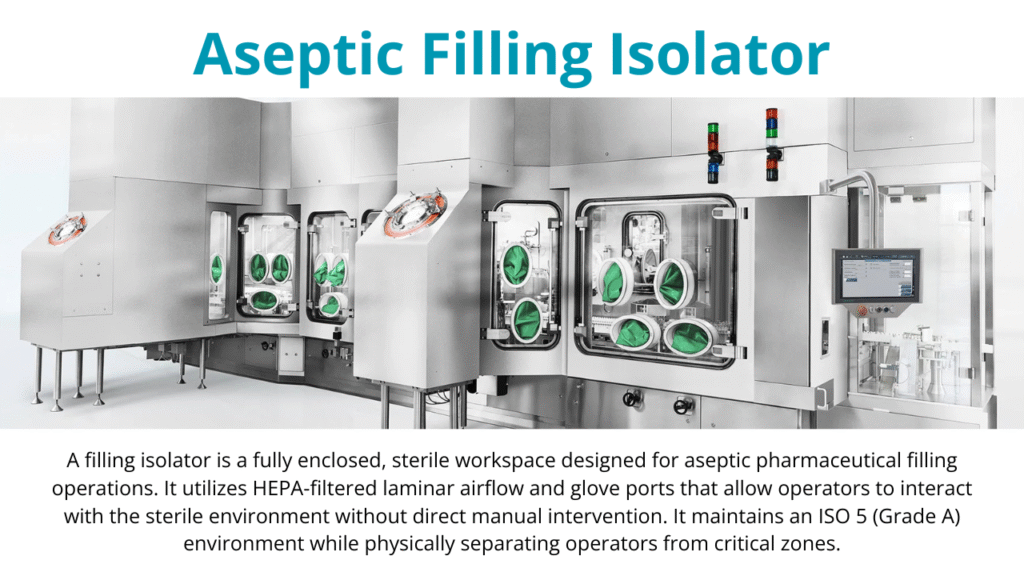
Why Aseptic Manufacturing is More Challenging?
Unlike terminal sterilization, where the final product is sterilized as a whole, aseptic processing relies on:
- Multiple sterilization steps (each component must be sterile).
- Environmental control (any breach in sterility can lead to contamination).
- Higher risk of human error (manual interventions increase contamination risks).
This approach to contamination control makes aseptic manufacturing more challenging than terminal sterilization methods. Due to these complexities, regulatory agencies such as the FDA and EMA prefer terminal sterilization whenever possible.
Aseptic processing is only permitted when terminal sterilization is not feasible.
Products Suitable for Aseptic Manufacturing
1. Protein and Peptide-Based Drugs
- Monoclonal antibodies (e.g., rituximab, trastuzumab)
- Insulin and insulin analogs
- Erythropoietin (EPO)
- Growth hormones (e.g., somatropin)
- Interferons (e.g., IFN-alpha, IFN-beta)
2. Vaccines and Biologics
- mRNA vaccines (e.g., COVID-19 vaccines like Pfizer-BioNTech and Moderna)
- Live-attenuated or inactivated vaccines
- Viral vector-based vaccines
- DNA vaccines
- Cell culture-derived biologics
3. Heat-Labile Antibiotics
- Penicillin and cephalosporin injectables
- Carbapenems (e.g., meropenem, imipenem)
- Vancomycin
- Some macrolides (e.g., erythromycin)
4. Cytotoxic and Chemotherapy Drugs
- Doxorubicin
- Paclitaxel
- Cisplatin
- Methotrexate
- 5-Fluorouracil
(These drugs degrade or form toxic by-products under high heat)
5. Ophthalmic Products with Sensitive APIs
- Preservative-free artificial tears
- Antibiotic/anti-inflammatory eye drops with unstable ingredients
- Combination formulations where at least one component is heat-sensitive
6. Biotech-Derived and Gene Therapy Products
- Cell and gene therapies (e.g., CAR-T therapies)
- DNA/RNA-based drugs
- Plasmid DNA formulations
- Viral vector suspensions (e.g., AAV, lentivirus)
7. Some Hormonal Preparations
- Recombinant human chorionic gonadotropin (hCG)
- Recombinant follicle-stimulating hormone (rFSH)
- Oxytocin (heat-sensitive when in solution)
8. Sterile Powders for Reconstitution
- Lyophilized (freeze-dried) antibiotics, peptides, and biologics
- Dry-powder vaccines
(These are aseptically filled into vials and later reconstituted before use)
9. Ophthalmic and Parenteral Suspensions
- Heat-sensitive suspensions (e.g., steroid suspensions)
- Micronized drug particles in sterile suspensions
(Terminal sterilization can alter particle size or consistency)
10. Complex Formulations with Emulsions or Liposomes
- Liposomal doxorubicin (e.g., Doxil)
- Emulsion-based parenterals (e.g., propofol)
- Nanoformulations used in cancer or antiviral treatments
Aseptic manufacturing is the only viable option for these products because terminal sterilization methods (such as autoclaving or irradiation) would alter their efficacy and stability.