The pharmaceutical equipment qualification process requires precision, quality, and adherence to regulatory compliance. Any deviation in equipment functionality can lead to product recalls, regulatory action, or worse, patient harm. That’s why the equipment procurement process is closely monitored and controlled. This entire process involves professionals from QA, QC, engineering, procurement, and project management.
In any pharmaceutical industry, purchasing equipment is not just about selecting a machine and using it. It’s a systematic process that involves several steps. These steps ensure the equipment is compliant with Good Manufacturing Practices (GMP), meets user needs, supports validation requirements, and fits into the quality management system. Any equipment, whether it’s a bioreactor, autoclave, or particle counter, must be carefully evaluated, tested, and documented. This article will guide you through the complete pharmaceutical equipment qualification process, from the initial URS to routine usage.
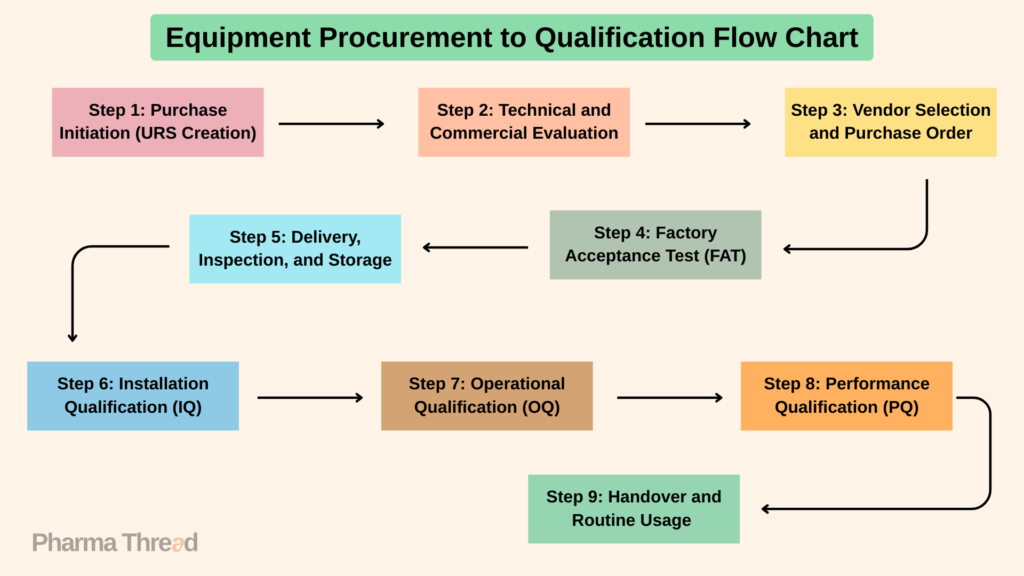
Step 1: Purchase Initiation (URS Creation)
The process begins when a user department—such as manufacturing, QC, or R&D identifies a need for new equipment. The team develops a User Requirement Specification (URS). This is one of the most critical documents. It includes the following specifications/needs that the user proposes.
- Intended use
- Equipment type and model preferences
- Functional requirements (capacity, speed, range, etc.)
- Compliance needs (e.g., 21 CFR Part 11, CE/GMP/ISO certifications)
- Utility and space requirements
- Cleaning and maintenance expectations
The user may collaborate with QA and engineering while preparing the URS to align expectations and regulatory requirements.
Step 2: Technical and Commercial Evaluation
After the URS is finalized, the procurement team initiates a Request for Quotation (RFQ) to multiple vendors. Quotations will be evaluated on technical and commercial aspects, which include the following.
- Compliance with URS
- After-sales service and AMC
- Warranty period
- Delivery lead time
- Installation support
- Training and documentation
- Price and payment terms
A cross-functional team, including the user department, QA, engineering, and procurement, evaluates the proposals.
Step 3: Vendor Selection and Purchase Order
The vendor offering the best value along with the best quality is selected. The purchase team will make the final negotiations, followed by the signing of contracts/agreements and issuance of purchase order. The purchase order (PO) should clearly mention technical specs, delivery timelines, FAT/SAT clauses, etc.
Step 4: Factory Acceptance Test (FAT)
FAT is a crucial step where equipment is inspected at the manufacturer’s facility before it is delivered to the customer. The purpose is to ensure that the equipment meets the specified requirements and functions as intended before installation. This is a pre-delivery inspection to ensure the equipment meets URS specifications before shipping.
However, FAT is not required for all equipment. FATs are particularly required for complex or critical equipment like process-specific equipment, software-controlled systems, large electrical equipment, etc. It is also required for the equipment that is custom designed to specific user requirements.
FAT involves:
- Visual inspection
- Basic operational checks
- Verification of safety features
- Preliminary calibration
- Document review (manuals, drawings, certificates)
This step saves time and costs by identifying issues before the equipment reaches the site.
Step 5: Delivery, Inspection, and Storage
Once the equipment is delivered at the site, the engineering or projects team checks:
- Packing condition
- Completeness of parts and accessories
- Physical damage or rust
- Calibration or MOC certificates
- Identification and labeling
Proper care must be taken while unloading and storing the equipment to ensure that the equipment remains undamaged before installation.
Step 6: Installation Qualification (IQ)
Installation Qualification (IQ) ensures the equipment is installed as per manufacturer and site requirements.
Key IQ checks:
- Equipment nameplate verification
- Utility connections (electrical, gas, water, etc.)
- Environmental and safety requirements
- List of supplied accessories
- Verification of documents: manuals, drawings, wiring diagrams
IQ is documented using an approved protocol, often jointly executed by the vendor and engineering/QA.
Step 7: Operational Qualification (OQ)
In this step, the equipment’s functionality is tested under controlled conditions but not under real production conditions.
OQ verifies:
- Control panel operations
- Alarms and safety interlocks
- Buttons and sensors
- Data recording and software functions
- Working range (e.g., temperature, pressure, etc.)
- Creating login credentials (Admin, Operator, QA, etc.)
- Verifying audit trials
All OQ tests are documented in a protocol with acceptance criteria and are signed off by QA and the user.

The Standard Operating Procedure (SOP) for the equipment must be prepared, reviewed, and approved before the start of Performance Qualification (PQ). The initial draft of the SOP may be prepared during installation qualification with the help of the user manual and diagrams. The SOP can be finalized during the operational qualification based on how the equipment works and what settings are required under operating conditions. Inputs from OQ are crucial for SOP completeness. The approved SOP must be in place for the operators to follow before performance qualification (PQ).
Step 8: Performance Qualification (PQ)
Now, the equipment is tested under actual operational conditions. In this step, real samples and real materials are used. However, the final product or final test result will not be of commercial use.
In the performance qualification, the following parameters will be monitored.
- System integration
- Environmental conditions
- Testing worst-case scenarios
- Process parameters
- Data integrity
- Alarm and error handling
- Reproducibility
- Product yield
Step 9: Handover and Routine Usage
Once PQ is completed and approved, the equipment will be handed over to the user department. QA ensures equipment is entered into the asset register, supporting documents, including IQ, OQ, PQ reports, calibration plan and training records are archived.
The equipment is now used in daily operations. QA, Engineering, and the User Department ensure:
- Routine usage logs are maintained
- Preventive maintenance is scheduled and performed
- Calibration is done at defined frequencies
- SOPs are followed for usage, cleaning, and maintenance.
- Requalification is planned post-maintenance or upgrades
The equipment must be in a validated state throughout its lifecycle.
Conclusion
Every step that we discussed ensures that the equipment is fit for its purpose, compliant with regulations, and ready to support pharmaceutical manufacturing and testing. Regulatory bodies like the USFDA, WHO, EMA, etc., expect compliance in the pharmaceutical equipment qualification, maintenance schedule, audit trials, and complete documentation and traceability. Following the structured approach mentioned above, companies can ensure regulatory compliance and safe pharmaceutical products.